Why do many drugs tested on animals then fail in clinical trials?

Clinical trials guarantee that human and animal patients receive medicines that are as safe as possible. No medicine can be 100% safe, i.e. have no side effects at all. Therefore, the tests on animals and humans are rigorous, so that medicines are developed that are as effective as possible with as few side effects as possible.
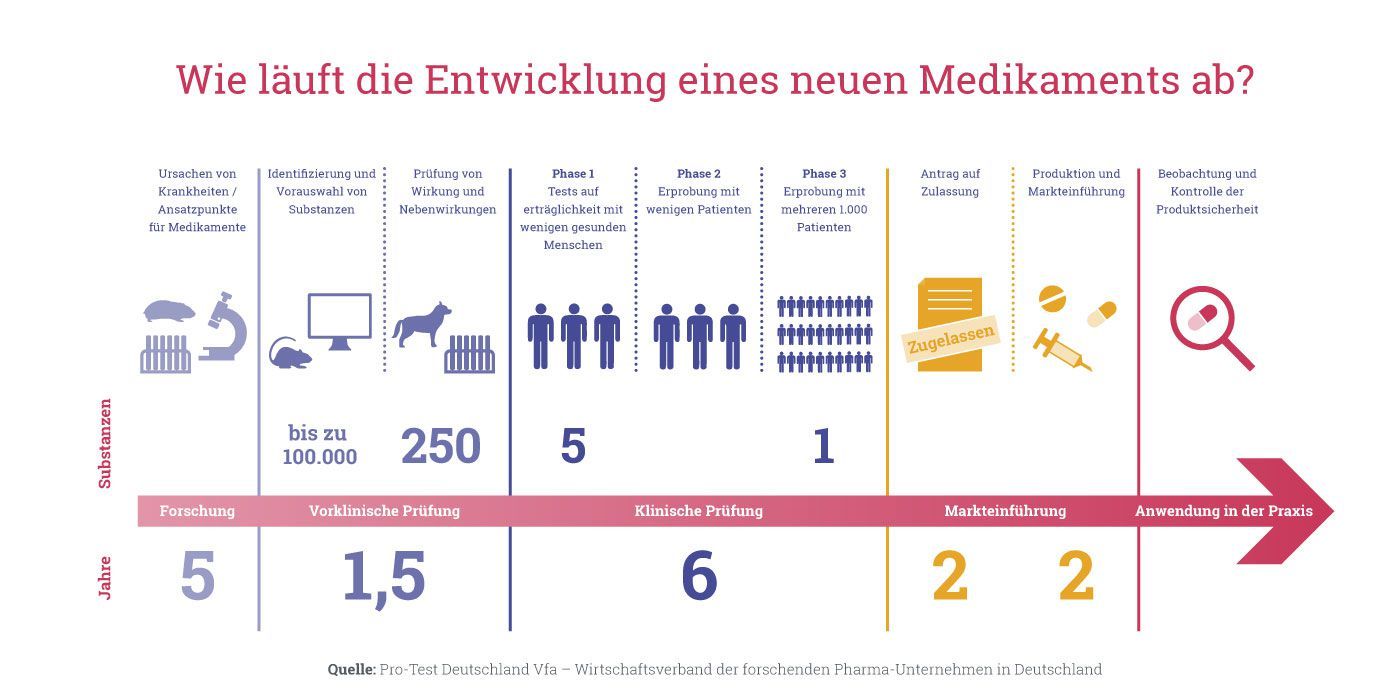
To do this, we must understand how these drug tests work: For a new compound to be approved and brought to market, it must go through several phases of testing. Initially, tests are only carried out on cell cultures and on laboratory animals. If they are successful, they are first tested on only a few people and – if the benefit is considered great and the risk low – gradually on more and more people.
In the preclinical safety tests (studies on animals), about a third of the experimental drugs already fail because they prove to be ineffective or too dangerous. Many substances then fail in the later phases of clinical trials for different reasons. That a preparation does not reach clinical trials means it was shown to be too dangerous for humans in studies on cell cultures or in animal experiments. This means the end for the further development of a preparation.
These regulatory phases are described in detail here; the further development of a drug depends on the successful completion of each phase:
Preclinical phase
Cell or tissue cultures are used to study how a substance works. If there are reliable indications that it is theoretically effective in an organism, animal experiments are carried out. Initially, small studies are started with very few animals. The questions that are answered there are: "Is there a possible therapeutic benefit?", "Is the compound safe enough to be used in Phase I in humans?". In addition, studies on the dose, effect, and distribution in the body (pharmacology and pharmacokinetics) are carried out in animals. In the following preclinical development, further animal tests are also carried out for toxicity tests to for example rule out malformations in foetuses. Most of these preclinical animal test studies are specified in terms of their size and research question by requirements from regulators such as Swissmedic or the American Food and Drug Agency FDA. The results are reviewed by the regulatory authorities, and clinical trials in humans are only permitted if approved by these authorities. A pharmaceutical company must therefore conduct animal studies if it wants to bring a drug to market, because they are required to receive a permission for clinical trials in humans.
Phase I
This is the first investigation in humans. This phase may only be started if the results in preceding animal tests were positive, and the substance is considered safe enough to attempt a first test in humans. Only a few human subjects are tested under strict conditions and monitoring to see if an active substance has a desired effect and is also safe for humans. These safety tests are usually conducted with six to ten healthy volunteers or on seriously ill patients for whom no other treatment options are available. Questions which researchers want to answer in phase I are: "What are the effects of the drug in humans?", "What are the side effects?", "Is a drug safe enough to study in a larger group of patients?".
Phase II
If enough evidence has been found in Phase I that a drug is beneficial and has not given rise to safety concerns, the drug can be moved to Phase II clinical trials. Now it is tested on a small number of selected patients. Depending on the type of disease the active substance is tested in a phase II trial on 20 to 300 test persons. Here, the focus is primarily on questions such as: "How does the treatment success depend on the dosage?" or "What is the most effective therapy regimen?".
Also in this phase, there are still animal studies conducted in parallel to check the effect of the drug on reproduction.
Phase III
If the results of phases I and II are encouraging, clinical phase III begins. This is the phase where a new drug is studied even more closely on a large group of patients under real-life conditions. Drugs are tested on 300 to 3000 or even more test subjects and usually compared to the standard therapy. Phase III is primarily focusing on questions such as: "Is the safety and efficacy of the new investigational drug better, worse or the same as comparable established treatments?", "How does the investigated active substance behave in combination with other drugs?" or "How can the drug best be prescribed to patients?".
In phase III, animal experiments are still running in parallel to exclude carcinogenic effects after long-term use.
Phase IV
After the successful completion of phase III, the drug can be approved by the regulators, but drugmakers will continue to monitor the drugs in clinical phase IV trials by recording and evaluating side effects. In everyday medical practice, a very large number of patients take the drug, so that a lot of data on efficacy and long-term effects can be obtained. This also makes it possible to identify very rare side effects.